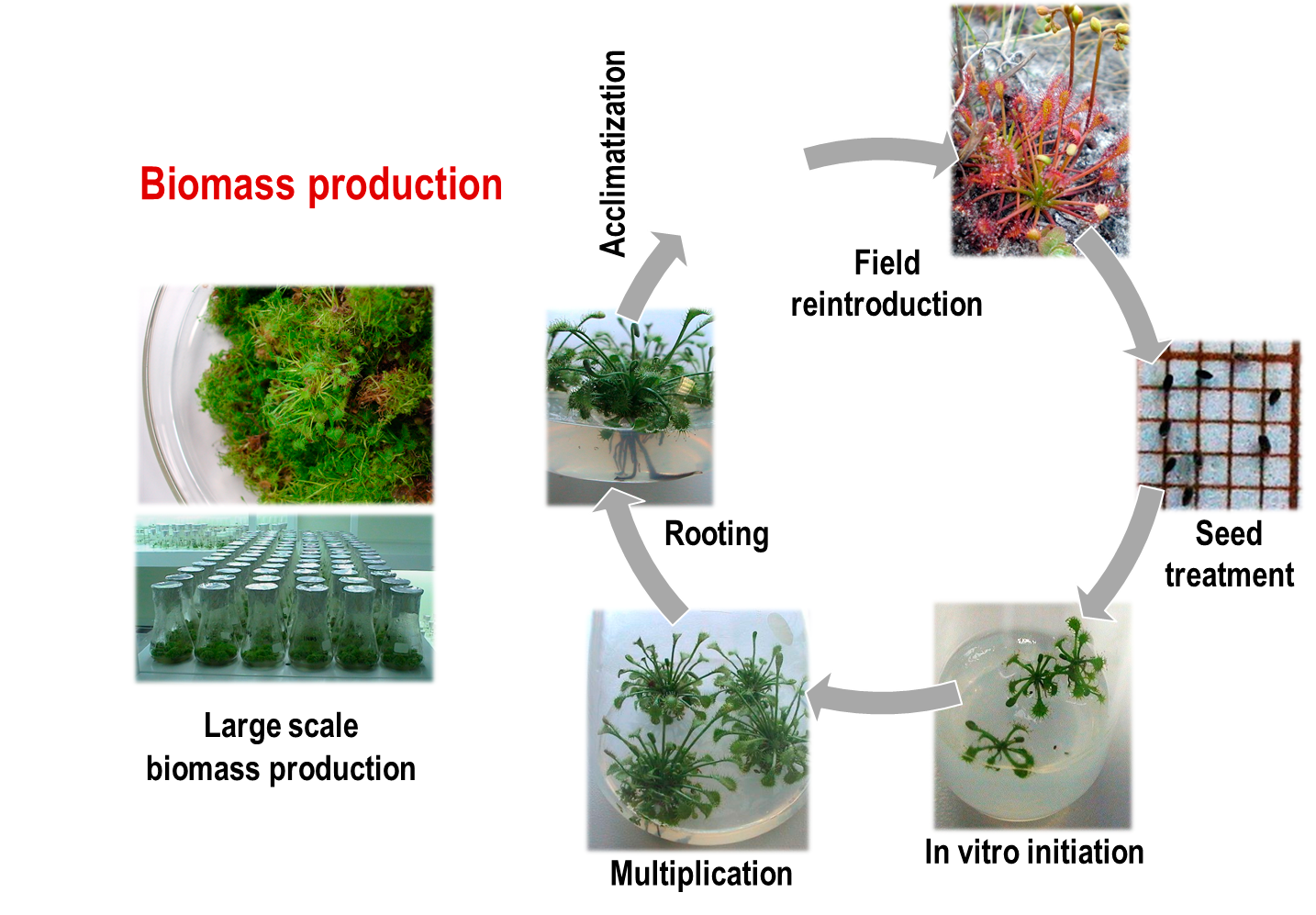
In vitro propagation of plants
Within this research line we develop plant propagation methods for wild endangered species medicinal plants and crops. This allows the large-scale propagation of healthy plants under controlled conditions and independently of seasonal variations.

Physiological and biochemical response of micropropagated plants to ex vitro transplantation and abiotic stress
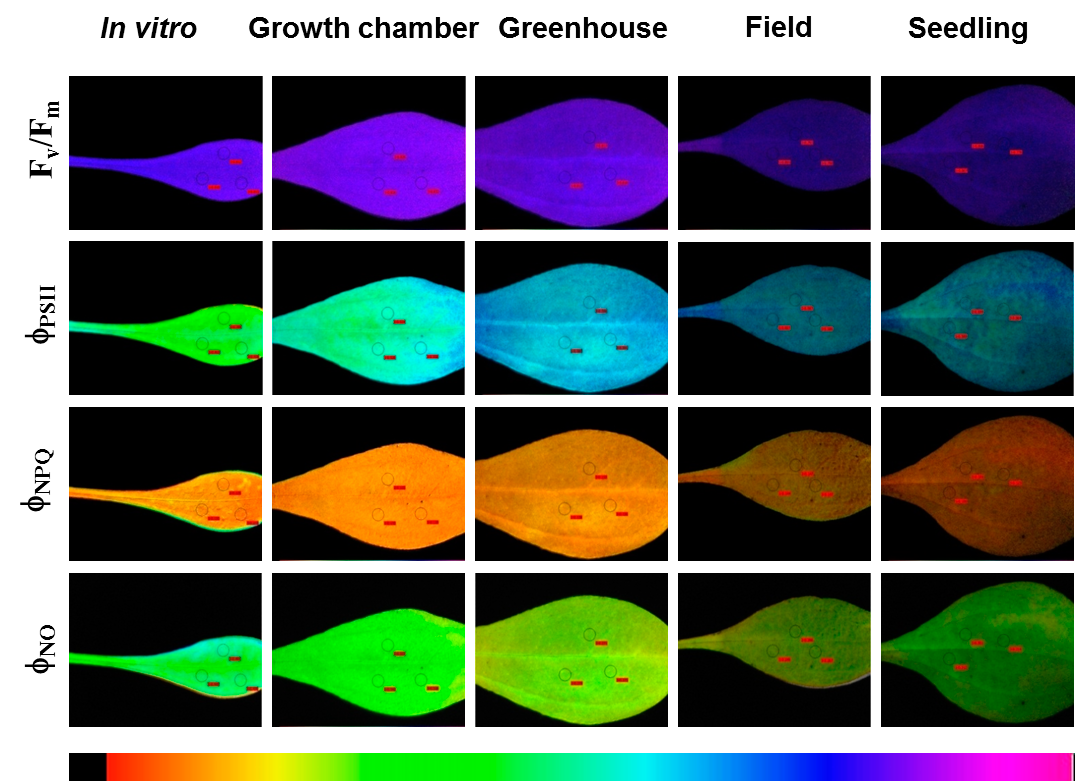
The in vitro growing conditions can be very stressful to plants leading to many physiological abnormalities and, consequently, to the overproduction of reactive oxygen species and oxidative stress that impairs their survival when transferred to ex vitro conditions. Understanding the oxidative stress status of micropropagated plants during acclimatization is of great importance to improve survival rates, plant development and the successful establishment under natural conditions.
We evaluate how micropropagated plants respond to new environmental conditions encountered during the acclimatization process by comparing their performance in the field in comparison with conventionally propagated plants. Several growth, physiological and biochemical parameters are evaluated in plans from both origins.
There is a great interest in understanding the physiological and molecular mechanisms that enable accumulator plants to take up and tolerate such remarkable amounts of metals. This knowledge could be useful for the selection of tolerant germplasm and for the development of plants with enhanced tolerance to metals and with increased metal uptake capability. Nevertheless, most metal accumulator plants are quite rare and often with a restricted distribution to threatened areas. In vitro propagation techniques can be an extremely valuable tool to surpass this constraint, permitting to evaluate plant response to abiotic stress without compromising their natural populations. We used in vitro produced plants of two rare species endemic from Portugal, Plantago algarbiensis Samp. and Plantago almogravensis Franco. to evaluate their aluminum (Al) bioaccumulation capacity, Al toxicity effects (growth, physiological traits and oxidative stress markers) and tolerance mechanisms. In vitro culture allowed obtaining rapidly extensive results regarding Al toxicity and tolerance mechanisms in two rare plants without compromising their natural populations.
Production of plant biomass for the extraction of active compounds
In vitro culture allows the rapid and mass production of plant material without a negative impact on natural habitats. Moreover, in vitro propagation methods allow the selective, rapid and effective production of secondary metabolites with no seasonal constraints and independent of geographical and environmental conditions.
In the Plant Biotechnology Lab we produced in vitro plant material of several plant species and investigate their chemical and biological profile in comparison with wild plant material.
Relevant publications:
Osório M.L., Coelho N., Gonçalves S., Osório J. & Romano A. 2013. Morpho-physiological traits and oxidative stress markers during acclimatization and field establishment of micropropagated plants of the endangered species Tuberaria major. Plant Cell, Tissue and Organ Culture, 115: 85-97. DOI 10.1007/s11240-013-0343-x
Martins N., Gonçalves S. & Romano A. 2013. Aluminum inhibits root growth and induces hydrogen peroxide accumulation in Plantago algarbiensis and P. almogravensis seedlings. Protoplasma, 250: 1295-1302. DOI: 10.1007/s00709-013-0511-1
Martins N., Osório M.L., Gonçalves S., Osório J. & Romano A. 2013. Impact of low pH and aluminum on the oxidative stress, energy partitioning and antioxidant responses in roots and leaves of Plantago algarbiensis and P. almogravensis. BioMetals, 26:427–437. DOI: 10.1007/s10534-013-9625-3
Martins N., Gonçalves S. & Romano A. 2013. Metabolism and aluminum accumulation in Plantago almogravensis and P. algarbiensis in response to low pH and aluminum stress. Biologia Plantarum, 57: 325-331. DOI: 10.1007/s10535-012-0271-3.
Costa P., Gonçalves S., Valentão P., Andrade P.B. & Romano A 2013. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L'Hér and their antioxidant and anticholinesterase potential. Food and Chemical Toxicology 57: 69–74.
Costa P., Gonçalves S., Valentão P., Andrade P.B., Coelho N. & Romano A. 2012. Thymus lotocephalus wild plants and in vitro cultures produce different profiles of phenolic compounds with antioxidant activity. Food Chemistry 135: 1253–1260.
Grevenstuk T., Gonçalves S., Domingos T., Quintas C., van der Hooft J.J.J., Vervoort J. & Romano A. 2012. Inhibitory activity of a naphthoquinone produced by Drosera intermedia on food spoilage fungi. Journal of the Science of Food and Agriculture 92: 1638-1642.
Grevenstuk T., Gonçalves S., Nogueira J.M.F., Bernardo-Gil M.G. & Romano A. 2012. Recovery of high purity plumbagin from Drosera intermedia. Industrial Crops and Products 35: 257-260.
Gonçalves S., Fernandes L. & Romano A. 2010. High frequency in vitro propagation of the endangered species Tuberaria major. Plant Cell, Tissue and Organ Culture 101: 359–363.
Grevenstuk T., Coelho N., Gonçalves S. & Romano A. 2010. In vitro propagation of Drosera intermedia in a single step. Biologia Plantarum 54: 391-394
Gonçalves S., Martins N. & Romano A. 2009. Micropropagation and conservation of endangered species Plantago algarbiensis and P. almogravensis. Biologia Plantarum 53: 774-778


