Germplasm conservation
Life on earth is dependent on plants, which are a crucial component of all ecosystems. Not only they are the basis of world food, but also can provide us fuel, clothes and medicine and play a major role in atmosphere and water purification and prevention of soil erosion. Plants are part of our natural heritage and it is our responsibility to preserve and protect them for future generations.
It is estimated that up to 100,000 plants, representing more than one third of all the world's plant species, are currently threatened or face extinction in the wild. In Europe, particularly, biodiversity is seriously threatened. Biotechnological approaches offer several conservation possibilities which have the potential to support in situ protection strategies and provide complementary conservation options.
The conservation of endangered plants using biotechnological techniques is an important research line at the Plant Biotechnology Lab. Within this line we develop protocols for micropropagation of endangered plant species like Thymus lotocephalus, Plantago algarbiensis and P. almogravensis, Tuberaria major, Drosophyllum lusitanicum, Rhododendron ponticum L.subsp. baeticum among other.
We develop new plant cryopreservation protocols for cryopreservation of seeds, shoot tips and assessment of genetic stability. Both micropropagation and cryopreservation are powerful tools in the ex situ conservation of plant genetic resources and together can provide means to replenish small populations and secure plant germplasm in cryobanks.
In vitro conservation
Plant propagation by tissue culture is an important biotechnological tool for the ex situ propagation of endangered plant species because it is possible to obtain thousands of plants from a single explant in a short period of time. The in vitro-cultured plants can subsequently be used for numerous different studies, avoiding collection from their natural environment.
Moreover, cultures can be maintained in vitro under slow growth conditions. Growth reduction is generally obtained by lowering the culture temperature, reducing or suppressing light intensity, applying growth inhibitors, or modifying the culture atmosphere.
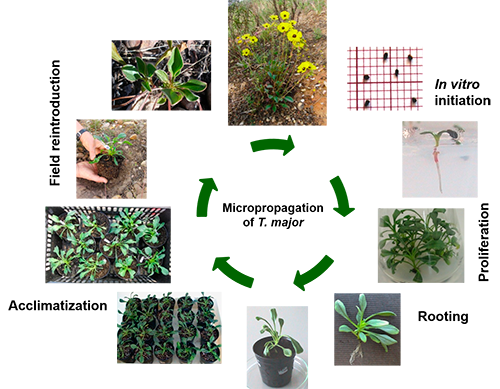
Figure. Micropropagation of Tuberaria major
Cryopreservation
Cryopreservation techniques are probably the most promising approach for preserving ex situ plant genetic resources and are of extreme importance when applied to plant species in danger of extinction. The ultralow temperatures of liquid nitrogen (-196ÂşC) allow the conservation of germplasm for a long period of time without deterioration, because at these temperatures all metabolic processes are drastically reduced. Different plant materials, such as seeds, shoot tips, nodal explants, cell suspensions, dormant buds and others, can be cryopreserved.
Plant germplasm stored in liquid nitrogen does not undergo cellular divisions. In addition, metabolic and most physical processes are stopped. Therefore, plant germplasm preserved under cryogenic storage can be maintained for very long periods of time and problems that are typical for storage in the active growth state, like genetic instability and the loss of accessions due to contamination, loss of vigour and totipotency and human error during continual subculturing, are overcome.
In new cryopreservation techniques, which can be used for the cryopreservation of shoot tips, plant material is dehydrated by exposure to highly concentrated cryoprotective solutions (vitrification procedures) and/or air desiccation (encapsulation-dehydration procedures), followed by rapid immersion in liquid nitrogen. The droplet-vitrification technique is derived from the droplet-freezing technique and the standard vitrification procedure based on the use of plant vitrification solutions. In this technique shoot tips are placed in small droplets of cryoprotective solution and cooled rapidly.

Figure. Cryopreservation of Plantago algarbiensis. Wild plant (bar: 7.5 cm approximately) (A). Seeds (B). Excised nodal segments 2-3 mm long (C). Seedlings from cryopreserved seeds (bar: 1 cm) (D). Plantlet from cryopreserved nodal segments by the droplet-vitrification method, eight weeks after culture (E).
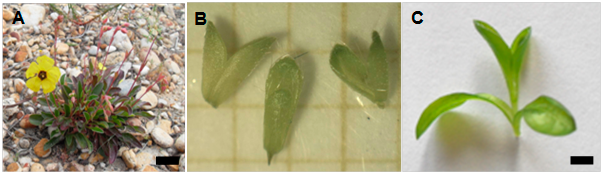
Figure. Cryopreservation of Tuberaria major. Wild plant (bar: 6 cm approximately) (A). Excised shoot tips approximately 2 mm long (B). Plantlet from cryopreserved shoot tips by the encapsulation-dehydration method, eight weeks after culture (bar: 0.5 cm) (C).
Seed conservation
Seed storage is the most efficient, easy and economical method for the ex situ preservation and, because each seed is genetically different, it allows the regeneration of whole plants from genetically diverse material. For these reasons, seed storage is the most widely used method of ex situ conservation. The conditions for conventional seed bank storage, for long-term storage, are drying at 10-25°C and 10-15% relative humidity to 3-7% moisture content, followed by storage at about -18°C. However, not all plant species can be preserved by this technique. Recalcitrant (desiccation-sensitive) and intermediate (relatively desiccation-tolerant) seeds cannot stand the desiccation conditions and cold storage without losing viability; only orthodox (desiccation-tolerant) seeds are able to do so.
An important problem in seed preservation of wild species is that many of them show seed dormancy and pre-treatments are needed to allow germination. Therefore, before the cryopreservation of seeds can be implemented as a routine technique it is important to study seed germination requirements and to demonstrate the feasibility of the technique.
Genetic analysis
For an effective implementation of any conservation programme, it is essential to assess the genetic diversity of natural populations. The information gathered can assist in the protection and management of populations and habitats, as well as in the strategy for germplasm collection, and genebank management. Molecular markers present a wide range of applications and are a useful tool in the study of genetic diversity of plant populations. Moreover, after cryopreservation it is necessary to assess the genetic integrity of plants surviving cryogenic storage to determine if they are 'true to type'. This can be done at the morphological, histological, cytological, biochemical and molecular levels.
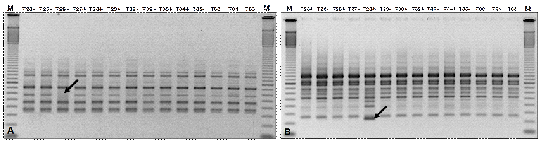
Figure. RAPD profiles of cryopreserved material and in vitro-grown shoots
Relevant publications:
Coelho N., González-Benito M.E. & Romano A. 2014. Approaches for the cryopreservation of Plantago algarbiensis, a rare endemic species of the Algarve. CryoLetters, 35: 521-529
Coelho N., González-Benito M.E. & Romano A. 2014. Cryopreservation of shoot tips from the endangered endemic species Tuberaria major. Acta Physiologiae Plantarum, 36: 3333-3336. Doi 10.1007/s11738-014-1678-6
Coelho N., González-Benito M.E., MartĂn C. & Romano A. 2014. Cryopreservation of Thymus lotocephalus shoot tips and assessment of genetic stability. CryoLetters, 35: 119-128.
Coelho N. 2014. Cryopreservation as a tool for preserving genetic variability of three endangered species endemic from Algarve region”, PhD Thesis, Universidade do Algarve, 171 pp.
Coelho N., Gonçalves S., González-Benito M.E. & Romano A. 2012. Germination and cryopreservation tolerance of seeds from the endangered species Thymus lotocephalus. Scientia Horticulturae, 145: 84-86. DOI: 10.1016/j.scienta.2012.07.031.
Martins N., Gonçalves S., Palma T. & Romano A. 2012. Seed germination of two critically endangered plantain species Plantago algarbiensis and P. almogravensis (Plantaginaceae). Seed Science and Technology, 40: 144-149.
Trindade H., Sena I., Gonçalves S. & Romano A. 2012. Genetic diversity of wild populations of Tuberaria major (Cistaceae), an endangered species endemic to the Algarve region (Portugal), using ISSR markers. Biochemical Systematics and Ecology, 45: 49-56. DOI: 10.1016/j.bse.2012.06.028.
Relevant publications:
Coelho N., Gonçalves S., González-Benito M.E. & Romano A. 2012. Establishment of an in vitro propagation protocol for Thymus lotocephalus, a rare aromatic species of the Algarve (Portugal). Plant Growth Regulation, 66: 69-74. DOI: 10.1007/s10725-011-9630-x.
Grevenstuk T. & Romano A. 2012. In vitro plantlet production of the endangered carnivorous Pinguicula vulgaris (Lentibulariaceae). Central European Journal of Biology, 7: 48-53. DOI: 10.2478/s11535-011-0103-z
Gonçalves S., Fernandes L. & Romano A. 2010. High-frequency in vitro propagation of the endangered species Tuberaria major. Plant Cell, Tissue and Organ Culture, 101: 359–363. DOI 10.1007/s11240-010-9683-y
Gonçalves S., Martins N. & Romano A. 2009. Micropropagation and conservation of endangered species Plantago algarbiensis and P. almogravensis. Biologia Plantarum, 53: 774-778. DOI: 10.1007/s10535-009-0142-8
Gonçalves S. & Romano A. 2007. In vitro minimum growth conservation of Drosophyllum lusitanicum. Biologia Plantarum, 51: 795-798. DOI 10.1007/s10535-007-0163-0.
Gonçalves S. & Romano A. 2005. Micropropagation of Drosophyllum lusitanicum (Dewy pine), an endangered West Mediterranean endemic insectivorous plant. Biodiversity and Conservation, 14: 1071-1081. DOI 10.1007/s10531-004-7846-z.


